0%
Target: ₹ 30,00,000 ($ 37500)
Raised: ₹ 0 ($ 0)
No. of Donors : 0
Completion Date: 31st December 2023
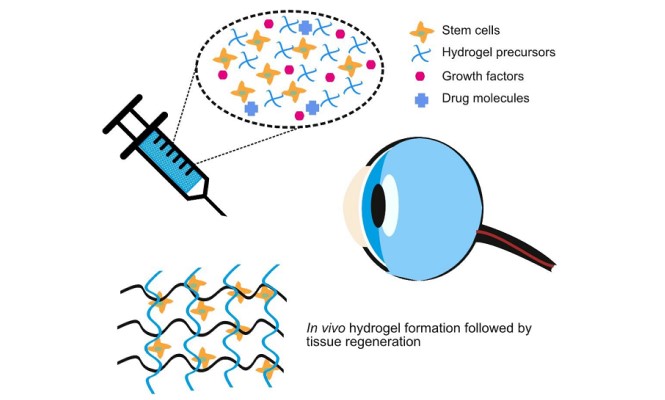
Decellularized_corneal_matrix_hydrogel-based_injectable_hydrogel-a_candidate_for_future_treatment_strategy_for_corneal_indications.pdf
Objectives of the project: A toxicology study will be essential to prove the safety of hydrogel during human applications.
- Preparation and characterization of Cornea hydrogel from Bovine sources
- Extensive Toxicology study
- Cytotoxicity Test
- Skin sensitization test
- Ocular Irritation in Rabbit
- Acute systemic toxicity test
- Material Mediated Pyrogenicity
- Intraocular irritation in Rabbits - 3 days
- Intraocular irritation in Rabbits - 90 days
- Maximum dose ocular irritation and persistence in rabbits - 14 days
- Data collection and documentation for Regulatory approval for human trial
Timeline and Budget:
Year 1: 15 Lakhs
Year 2: 10 Lakhs
Year 3: 5 Lakhs
Proposer Name & Designation:
Dr. Falguni Pati, Associate Professor, Department of Biomedical Engineering







